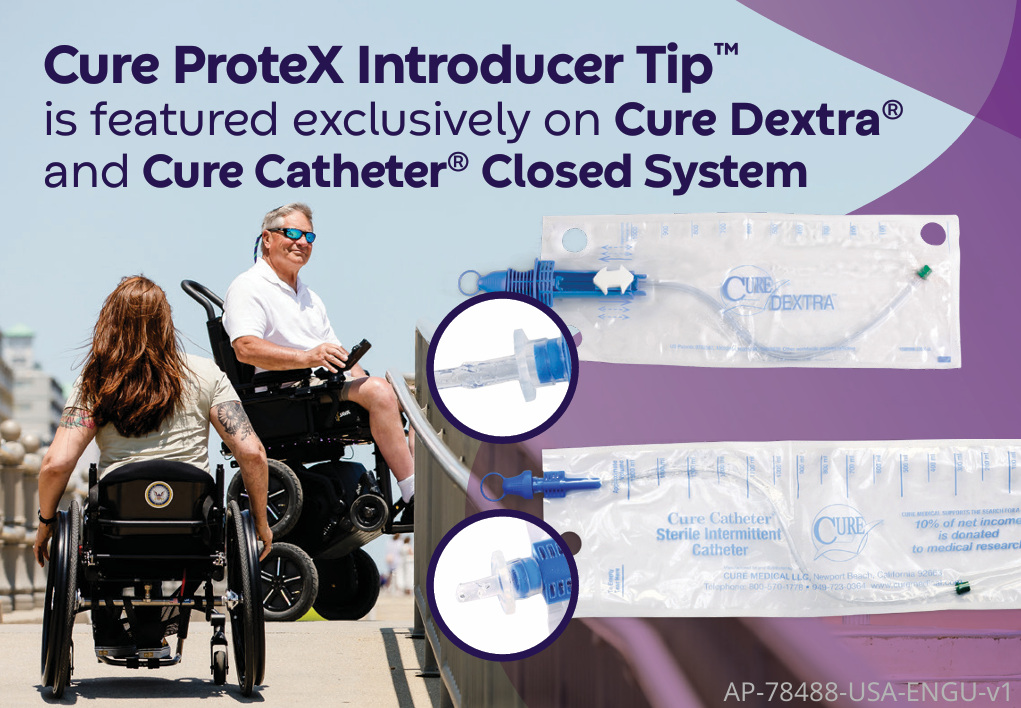
Cure ProteX Introducer Tip™ offers advanced protection
The pre-lubricated Cure ProteX Introducer Tip™ is designed with an X-shaped opening in an enclosed, pre-lubricated tip and has been shown to provide advanced protection against bacteria transfer.1 The Cure ProteX Introducer Tip™ is featured exclusively on Cure Dextra® and Cure Catheter®Closed Systems.
Cure ProteX Introducer Tip™ is designed to help increase independence
A published research paper presenting results of in vitro testing reported NO bacteria was found at the end of the simulated urethra and finds the Cure ProteX Introducer Tip™:
- REDUCES bacterial displacement with bacterial numbers at undetectable levels¹
- PROVIDES an effective bacterial barrier
- OUTPERFORMS three other leading intermittent catheter brands in every test
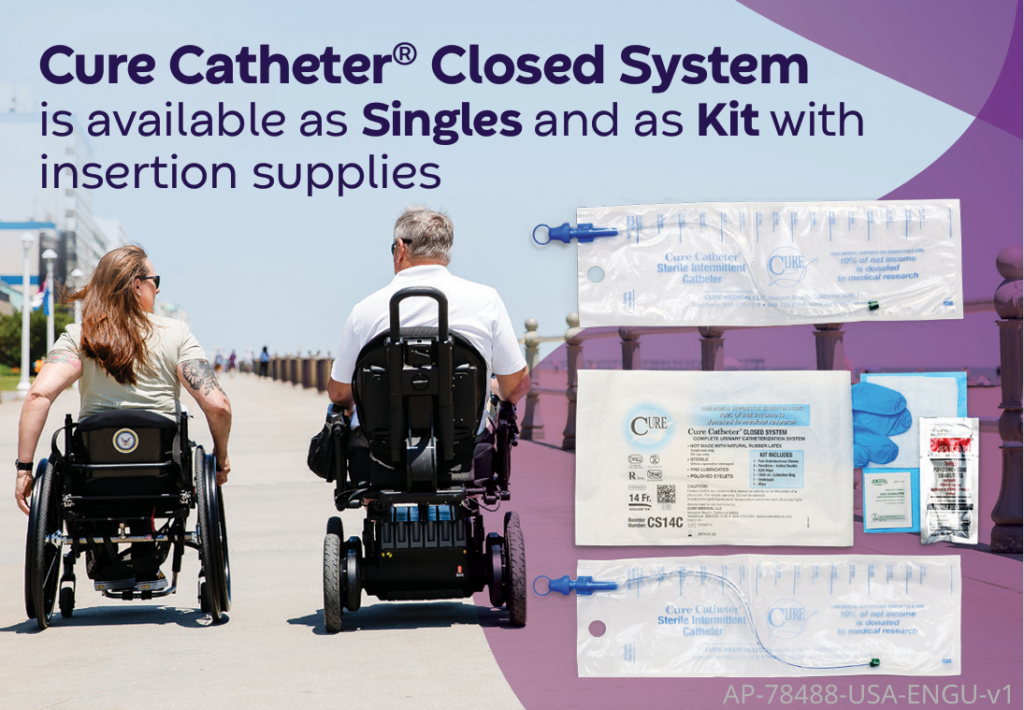
Like all Cure Medical catheters, the Cure Dextra® and the Cure Catheter® Closed System feature smooth, fire polished eyelets for comfort. They are not made with DEHP/DINP, BPA or NR-Latex for safety.
**Effective January 1, 2026, CMS announced that Medicare‑insured individuals with spinal cord injury who use intermittent catheters are now eligible to receive closed system catheters, regardless of level of injury.

About Convatec:
Convatec Continence Care manufactures innovative intermittent catheters for men, women, and children. Advanced technologies and proprietary features are designed to provide a safe, comfortable, and convenient cathing experience—while helping to reduce the risk of infection. Products are not made with DEHP/DINP, BPA, or NR-Latex. From closed systems to hydrophilic, pre-lubricated/ready-to-use, and uncoated styles—you can be confident in your choice.
References: 1. Meredith K, Pollard D, Mason V, Ali A with Convatec Ltd, Evaluation Of Bacterial Displacement By Intermittent Catheters With Gel- Or Water-Based Lubricants, An In-vitro Urethral Agar Channel Model. Continence 12S (2024) 101590 DOI: 10.1016/j.cont.2024.101590 | AP-75832-USA-ENGU-v1